One of the axioms in the search for life beyond Earth has been “follow the water.” Perhaps scientists should broaden that to include frozen water, or ice, as recent experiments suggest space ice harbors intriguing properties.
◊
Astrobiologists have had a tough gig. With very little to put under their microscopes in the lab, they were forced to pore over spectral analysis studies from telescopes looking into deep space. Occasionally they’ve gotten to dissect a piece of meteorite with promising signs of organic chemistry. In one instance, a Martian meteorite found in the Allan Hills region of Antarctica controversially contained microscopic fossils of what appear to be bacteria. The jury is still out on whether it’s actually a sign of life.
Nowadays, our robotic space probes are returning a wealth of imagery, spectral information, and more. In fact, a couple of probes have collected samples of cosmic dust and returned samples from the surfaces of comets and asteroids. Results show that icy comets are covered in organic molecules, including carbon-hydrogen or carbon-carbon bonds like methane, ethane, and other hydrocarbons.
The Rosetta mission returned a sample from comet 67P-Churyumov-Gerasimenko. The sample contained an amino acid, glycine, which is a part of proteins and a key component in cell membranes and even in DNA.
How do these complex molecules form in the cold reaches of space? If not liquid water, what other mechanism could do the job?
For more on space ice, check out this episode of the MagellanTV original series Space: The New Frontier.
Organic Soup
Onboard cameras and sensors are returning fantastic imagery of the ice moons of the gas giants (Jupiter, Saturn, Neptune, Uranus); Pluto; and other planetoid objects in the asteroid belt. One thing that is noticeable even to the layperson is that everywhere there is ice you will find a reddish-brown stain on the surface or in the cracks of ice sheets. (Pluto and Jupiter’s moon Europa are fine examples of orbs with this “stain.”)
Though this red stuff was named ‘tholins’ by legendary astrophysicist Carl Sagan and his associate Bishun Khare, it is more popularly known as “brown sticky gunk” that they had recreated in the laboratory. Tholins consist of a lot of carbon compounds or organic molecules formed by simple compounds like carbon dioxide and methane.
Irradiated with cosmic rays or ultraviolet light, tholins are helped along with water and nitrogen. It doesn’t take much for them to build up more complexity, longer chain molecules, nucleic acids, proteins, etc., until you have everything needed to create single-cell life.
Some astrobiologists believe it may be a natural process in the formation of stars and their planetary bodies for interstellar dust essentially to build complexity, and eventually life, wherever it can gain a foothold.
Pond Scum
As with any profession, experts frequently hold differing views. As for the origins of bacterial life, some favor the undersea thermal vents theory, and others favor the surface hot springs model. The Darwinian pond scum scenario is the one I favor. To build long-chain molecules you need a wetting and drying phase – like the ebb and flow from a hot spring – and you can’t get that under the ocean.
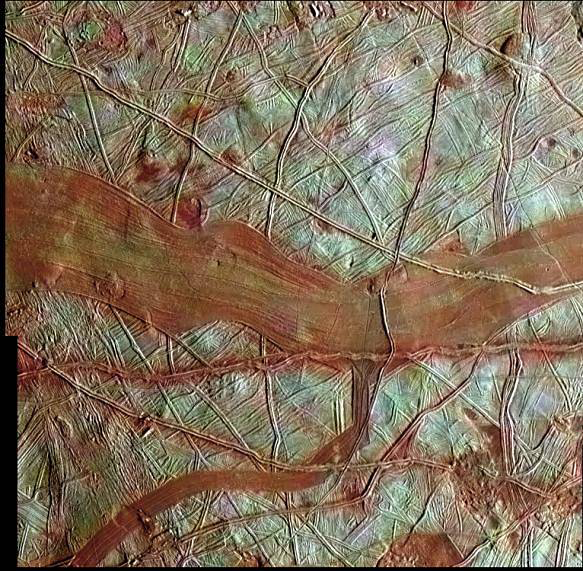
Jupiter’s moon Europa shows tell-tale signs of the buildup of tholins. (Source: NASA/JPL/Caltech/SETI Institute)
So how do we examine these organic molecules in the icy depths of space? One test used neutron beams from a nuclear reactor to study ices on the moons around Jupiter and Saturn. The results indicated that the ice molecules formed complex micro shapes, tubes, and compartments that in theory could be filled with organic elements that would bind together – sort of a manufacturing plant. I found that very interesting.
Then I read about some new discoveries in the laboratory dealing with ice. H2O is quite an interesting and special molecule. I won’t bore you with unnecessary details about water, except that it can exist in several states. It changes from a flowing state to a crystalline structure that expands in volume and floats in liquid water – what we call ice.
Amorphous Ice
Ice in space is very different from ice on Earth. It's called amorphous ice. This ice doesn’t have a crystalline structure like ice in your lemonade. Its structure is haphazard, like liquid water.
Amorphous ice is created by the very rapid cooling of water, so it doesn’t have time to form the usual lattice structure. (One would expect this to happen in the vacuum of space.) Another way to make it is to compress normal ice at very low temperatures, as might happen on the surface of an ice moon.

Artist’s impression of the ice-covered surface of Pluto. (Source: SA/Hubble [L. Calçada])
There are 20 crystalline forms of ice known so far. There are high- and low-density amorphous ices and, just recently, a medium type was produced in a lab. When warmed up, this ice re-crystallizes and releases a lot of heat energy. Without the normal crystalline lattice structure, this ice behaves like water and can actually flow, but in very slow motion.
Two forms of this ice-water can also coexist like oil and water, and move independently. So, we have icy moons orbiting large planets. The gravitation effects squeeze and pull at a moon, causing tectonic movement, generating heat, and melting or freezing these various ices over a long period of time. Might this supply the necessary wetting and drying phases to build longer chain molecules?
This amorphous ice could be the mechanism for forming complex organic molecules (at least to the “tholins” stage, as seen on Pluto) in the most inhospitable environments. Perhaps Europa evolved the early stages of life on the surface and in the cracks of its icy surface, which then drifted down to the ocean floor beneath where thermal vents mix water with the dissolved minerals from the rocky bottom, feeding extremophiles.
As seen in the returned cometary samples, the complexity could increase to include amino acids. If we took more samples from around the Solar System, would we find more amino acids, proteins, or monomers – or sugars, the building blocks of carbohydrates?
Of course, all this chemical alchemy takes a very long time, billions of years. Might it be that this chemistry quickens in ideal environments like Mars, Earth, and Venus?

The photo of Pluto's surface shows where some scientists believe they may find organic material. (Source: NASA/JPL)
Titan: A Complex Environment with a Promising Bio-signature
Another scenario well worth investigating is the enigmatic Titan, Saturn’s largest moon. Titan is one and a half times bigger than our own Moon. It has a thick atmosphere and is known to have lakes of liquid hydrocarbons. Based on an icy crust, the planet is covered in complex molecules and has a weather cycle not dissimilar to our own. It has an atmosphere composed mostly of nitrogen discolored by tholins and clouds of ethane and methane. Hydrocarbon rain erodes the surface much like water rain on Earth but at a much lower temperature – minus 180 Celsius.
One could imagine this would be an ideal locale to increase the complexity of these long-chain molecules and form amino acids and, eventually, bacterial life. We will find out soon enough, as NASA is sending a robotic quadcopter to Titan. The Dragonfly mission will fly around the surface and take samples, looking for both prebiotic chemistry and, if possible, life signs.
The conclusion could be that there will be precursors for life everywhere in one form or another, around every planetary system, from deep in the outer debris fields like the Oort cloud through to the Goldilocks Zone and further in to a Venus-type planet. Based on this idea, you would definitely find signs of bacterial life, ancient or otherwise, on Mars, the ice moons, including Titan, and perhaps in the atmospheres of the gas giants and even Venus.
We just have to wait patiently, perhaps for a billion years or so.
Ω
Living in Melbourne, Australia, Andrew Thomson has run his own production company, Astro Media, since 2004, producing original documentary programs for the international market. He is an animal lover and, for a hobby, has studied Paramedical Science. He occasionally works night shifts onboard an ambulance, looking to develop some sort of empathy for humans.
Title Image: The icy surface of Pluto, captured by NASA’s New Horizons spacecraft, is rich in nitrogen, carbon monoxide, and methane ices. (Source: NASA/JHUAPL/SwRI)

